Research
Expertise
Multi-parameter flow cytometry, spectral flow, & CyTOF
Biometabolism
Murine models of human disease
High-throughput sequencing (ATAC, ChIP, FAIRE)
Experience with primary human lymphoid cells
IACUC / IRB protocol writing and revision
Fields of Interest
Cellular & molecular immunology
Epigenetics and gene expression
Solid organ and HSCT transplant immunobiology
Scholarship of teaching and learning, discipline-based education research, educational psychology, metacognition
Current Work
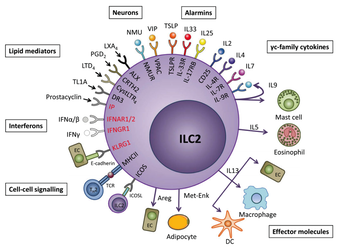
I am a fourth year postdoctoral fellow in the Duke-UNC Immunotherapy Training Program at the University of North Carolina. I work in the laboratory of Dr. Jonathan Serody at the UNC Lineberger Comprehensive Cancer Research Center, where I am investigating epigenetic regulation of type II innate lymphoid cells in acute graft- versus-host disease following hematopoietic stem cell transplantation.
Multi-parameter flow cytometry, spectral flow, & CyTOF
Biometabolism
Murine models of human disease
High-throughput sequencing (ATAC, ChIP, FAIRE)
Experience with primary human lymphoid cells
IACUC / IRB protocol writing and revision
Fields of Interest
Cellular & molecular immunology
Epigenetics and gene expression
Solid organ and HSCT transplant immunobiology
Scholarship of teaching and learning, discipline-based education research, educational psychology, metacognition
Current Work
I am a fourth year postdoctoral fellow in the Duke-UNC Immunotherapy Training Program at the University of North Carolina. I work in the laboratory of Dr. Jonathan Serody at the UNC Lineberger Comprehensive Cancer Research Center, where I am investigating epigenetic regulation of type II innate lymphoid cells in acute graft- versus-host disease following hematopoietic stem cell transplantation.
Grants & funding
09/2021 - Present: DUNC-IMTG T32 Fellowship (Co-PIs: Drs. Jonathan Serody & Nelson Chao)
Duke University, Durham, NC
University of North Carolina at Chapel Hill, Chapel Hill, NC
NCI: T32CA211056-01A1
09/2018-08/2021: NIGMS IRACDA SPIRE Scholarship (PI: Dr. Donald Lysle)
University of North Carolina at Chapel Hill, Chapel Hill, NC
NIGMS: K12GM000678-20
2014-2018: NRSA Ruth L. Kirschstein National Research Service Award (F31 PI: Sonia Laurie)
Emory University School of Medicine, Atlanta, GA
NIAID: F31AI114250-01
2014: Recipient of NIAID RO1 Sub-Award (PI: Dr. Mandy L. Ford)
Emory University School of Medicine, Atlanta, GA
NIAID: RO1AI104699-01A1
2013-2014: Emory University NIH T32 Institutional Training Grant (PI: Dr. Brian D. Evavold)
Emory University School of Medicine, Atlanta, GA
NIAID: T32AI007610-15
2009-2012: Post-baccalaureate Research Experience Program Scholar (PI: Dr. Henry H. Wortis)
Tufts University School of Graduate Biomedical Science, Boston, MA
NIGMS: GM066567-16
Duke University, Durham, NC
University of North Carolina at Chapel Hill, Chapel Hill, NC
NCI: T32CA211056-01A1
09/2018-08/2021: NIGMS IRACDA SPIRE Scholarship (PI: Dr. Donald Lysle)
University of North Carolina at Chapel Hill, Chapel Hill, NC
NIGMS: K12GM000678-20
2014-2018: NRSA Ruth L. Kirschstein National Research Service Award (F31 PI: Sonia Laurie)
Emory University School of Medicine, Atlanta, GA
NIAID: F31AI114250-01
2014: Recipient of NIAID RO1 Sub-Award (PI: Dr. Mandy L. Ford)
Emory University School of Medicine, Atlanta, GA
NIAID: RO1AI104699-01A1
2013-2014: Emory University NIH T32 Institutional Training Grant (PI: Dr. Brian D. Evavold)
Emory University School of Medicine, Atlanta, GA
NIAID: T32AI007610-15
2009-2012: Post-baccalaureate Research Experience Program Scholar (PI: Dr. Henry H. Wortis)
Tufts University School of Graduate Biomedical Science, Boston, MA
NIGMS: GM066567-16
Early Career
My early research focused on enhancing our understanding of the genetic mechanisms underlying metabolic and immune disease. In my undergraduate research experience I worked to define and compare the effects of a high fat diet in salt sensitive rats that contained consomic chromosomal substitutions from resistant Brown Norway (BN) rats to determine which chromosome conferred resistance to metabolic syndrome. Our results indicated that chromosome 8 substitution rats possess a trait that protects them from elevated levels of cholesterol, triglycerides, and glucose while on a high fat diet. Our findings were published in The Journal of Nutrigenetics and Nutrigenomics in 2012. After graduation, I accepted a two year NIH-funded post-baccalaureate fellowship at Tufts University, where my project involved characterizing the role of T lymphocytes in protection against intraerythrocytic parasite infections. I established that MHC class II molecules are absolutely required to clear infection with the bloodborne protozoan parasite Babesia microti. My findings suggest that the IL-21 receptor is necessary for protection and that phagocytosis is an indispensible component of acute resistance to infection. My findings indicated that the differential parasite burden observed in resistant BALB/c and susceptible DBA/2J mice may be due to a difference in IFN-γ production, although follow-up studies are needed. Additionally, I helped to establish two congenic lines of resistant mice that have subsequently been used in mapping studies to identify alleles responsible for protection against age-acquired susceptibility to babesiosis.
Graduate Work
My dissertation work focused on understanding the mechanisms by which T lymphocytes mediate cellular rejection of allografted tissues following solid organ transplantation. Immunotherapeutic strategies to prevent rejection following transplantation frequently involve targeted blockade of T cell costimulatory pathways. My project assessed the contribution of coinhibitory receptors to the control of alloreactive T cell responses following transplantation in humans and animal models. I demonstrated that the T cell coinhibitory molecules 2B4 and TIGIT are expressed on CD28null effector memory CD4+ and CD8+ T cells that are associated with freedom from rejection following renal transplantation in humans. Further exploration of these pathways in a murine model of transplantation indicates that while 2B4 functions to control alloreactive T cells by limiting their glycolytic metabolism, proliferation and recruitment into the alloreactive anti-donor response. Finally, my work demonstrated that the programming of antigen specific CD8+ T cells responding to graft and pathogen are dissimilar, and that antigen-specific CD8+ T cells primed by a skin graft contract faster than those primed by infection, yet are able to expand more rapidly upon rechallenge. These data suggest that graft-elicited CD8+ antigen specific T cells are maintained in a less terminally-differentiated state compared to pathogen-elicited CD8+ antigen specific T cells. Taken together, these data suggest that the surface marker expression, metabolic prolife, and functional capacity of T cells depends on the priming conditions and may be used to predict immunologic risk following transplantation. In addition to the contributions I made to the projects above, I was also been involved in training and mentoring a number of undergraduate, graduate, and post-graduate research scientists at the Emory Transplant Center.
Post-doctoral work
As a postdoctoral fellow, my research has focused on hematopoietic stem cell transplantation (HSCT), the preferred treatment for a variety of blood malignancies. Unfortunately, its use is limited by the development of acute graft-versus-host disease (aGvHD). Type II innate lymphoid cells (ILC2s) are immune cells that play an important role in maintaining mucosal homeostasis, and our lab has previously shown that ILC2s in the gastrointestinal tract (GI) are sensitive to conditioning therapy prior to HSCT. Strikingly, we have demonstrated that the infusion of activated donor ILC2s markedly reduces aGvHD-associated mortality. My specific work serves to further investigate the mechanism of the loss of protective ILC2s from the GI tract. Taken together, this work will provide new insights into mechanisms by which innate lymphoid cell precursors are epigenetically regulated, providing novel approaches to treating aGvHD following HSCT.